Optimizing Crystal Growth Using Thermal Analysis

Thermal analysis helps you develop a road map for synthesis and crystal growth of novel materials
Daniel G. Chica, PhD.
Chemistry Department
Thermal analysis is a crucial tool for the solid-state synthetic chemist. This technique allows for the determination of the precise temperature at which the system releases or absorbs heat corresponding to melting, crystallization, and structure changes, among other thermal events. Therefore, heating profiles can be effectively tuned to optimize the synthesis of materials that ensures phase purity, compositional homogeneity, and high crystallinity in a timely matter. In the context of liquid or vapor phase, single crystal growth, knowledge of these aforementioned thermal events are absolutely necessary as optimal crystallization usually occurs in a narrow temperature range.
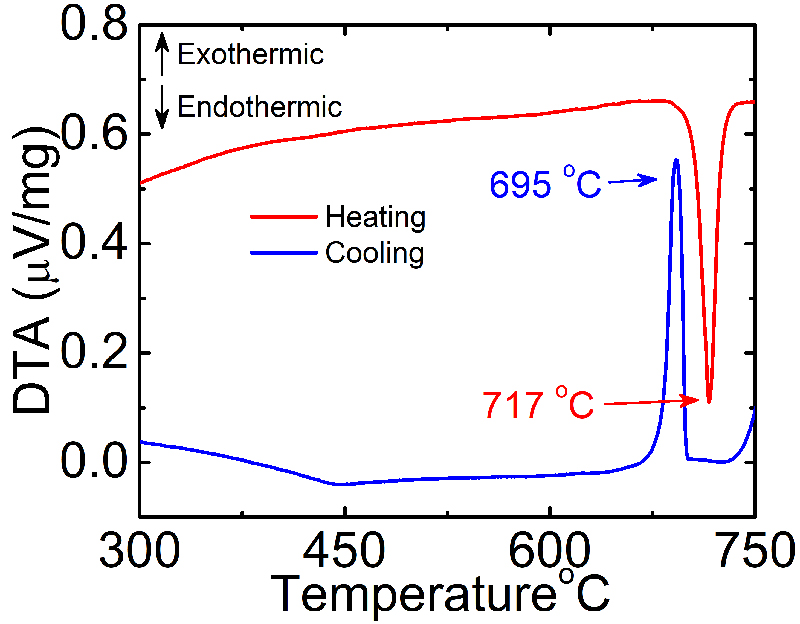
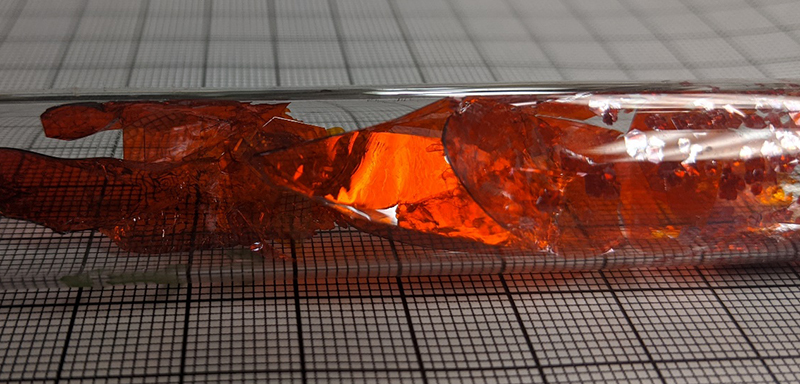
For my research, I used differential thermal analysis (DTA) to determine the melting and crystallization temperature of LiInP2Se6, a promising material for the detection of thermal neutrons. Since high quality, single crystals of this material is a prerequisite for use as a radiation detection, I employed a chemical vapor transport reaction to grow single crystals. For this reaction to work well, the reaction must proceed below the melting point to balance the vapor pressures of the gas phase, transport effective species. I was able to grow crystals with areas of cm2 ’s and thicknesses from 50μm – 1mm. Under irradiation of thermal neutrons, LiInP2Se6 detectors produced a signal distinct from the background.